April 2022 - Newsletter
Included in this newsletter
- Audits and Inspections Are Back with Vengeance
- COVID Standing Orders: Updated
- Orthoses- Prior Authorization Suspended
- Updated and New Policies, Procedures, and Forms
- Medicare Beneficiaries Get Free OTC COVID-19 Tests
- Compliance Portal® Feature – License Verification
- CBD Expert Advice for Getting Started
- R.J. Hedges Bulletin Releases/Webinars

Audits and Inspections Are Back with Vengeance
Over the last month, we have seen a drastic increase in regulatory inspections. State boards and DEA are actively in pharmacies and small healthcare practices. DEA has issued four fines in the last week, all over $70,000. The Office of Civil Rights (OCR) also began inspecting small healthcare practices on HIPAA Breaches or spot inspections. The OCR also issues fines for non-compliance both on breaches and the spot inspections. Several chiropractors and dentists have fallen out of grace with the OCR and received fines.
CMS has resumed its RAC Audits on Part B pharmaceuticals and DMEPOS products. These audits require more documentation than pre-pandemic requirements. Remember, the entire Medicare Part B documentation requirement changed on January 1, 2020. State Department of Health inspections go into the pharmacies and review the COVID-19 requirements. This is a new inspection type adding to the pile. The ones we have been involved with the inspectors are reading the policies and procedures, standing orders, and other immunization requirements. Some of these inspections take up to three hours to complete.
Insurance audits by OptumRx, CVS/Caremark, and MedImpact are more aggressive in their documentation requirements.
The good news is if you are completing the monthly task lists each month and understand how to navigate the Compliance Portal®, these audits and inspections are not a real issue.
COVID Standing Orders: Updated
The COVID-19 pandemic is winding down. It is time to stop using the waivers for patient signatures. Patients need to begin signing the OBRA Log, signature capture system, and the Receipts of Goods and Services. We expect these waivers and mandates to end on April 18th; however, with the CDC’s extension for 90 days, it is time to make these operational changes regardless of the CDC or HHS. Your staff will need to be re-trained for all of the signature requirements through the operation.
COVID-19 Standing orders continue to change rapidly as new variants develop. Please check the IAC standing orders weekly to make sure you are using the newest version for all the COVID-19 standing orders. For the newest IAC version, click on the standing order found on the portal. It will take you directly to the standing order. The date is found at the bottom left-hand corner.
Orthoses- Prior Authorization Suspended
Due to the need for certain patients to receive an orthoses item that may otherwise be subject to prior authorization when the two-day expedited review would delay care and risk the health or life of the beneficiary, we are suspending prior authorization requirements for HCPCS codes L0648, L0650, L1832, L1833, and L1851 are furnished under these circumstances:
- Claims for these HCPCS codes that meet the above description will be billed using modifier ST and will not undergo prior authorization. These claims will instead be subject to a 100% prepayment review.
- For suppliers furnishing these items under a competitive bidding program exception (as described in 42 CFR 414.404(b)), claims billed with modifiers KV, J5, or J4 would convey that the DMEPOS item is needed immediately. Therefore these modifiers will be accepted in addition to the ST modifier. Ten percent of claims submitted using the KV, J5, or J4 modifiers for HCPCS L0648, L0650, L1833, and L1851 will be subject to prepayment review.
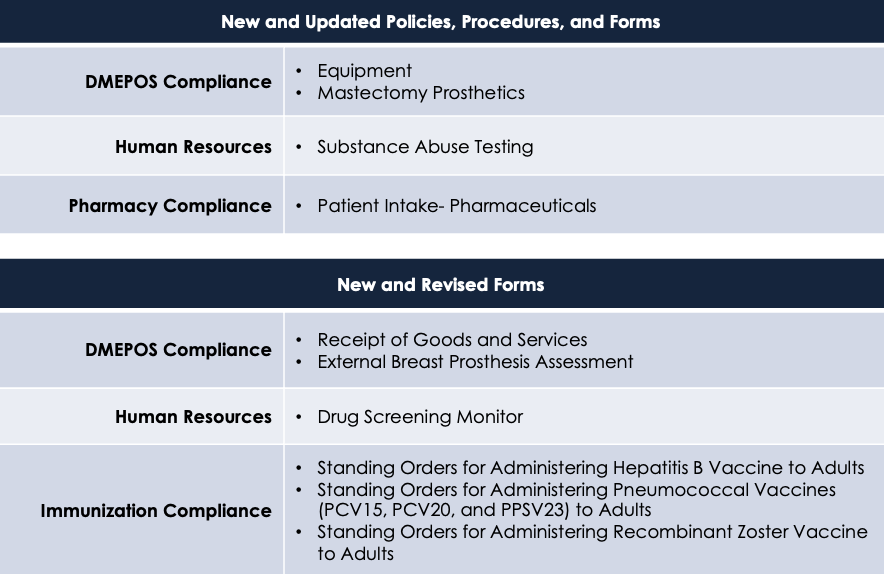
Updated and New Policies, Procedures, and Forms
There are a number of rules, regulations, and guidelines that are about to change. Please see the table below for the areas and changes that will be made.
Medicare Beneficiaries Get Free OTC COVID-19 Tests
On April 4, The Biden-Harris Administration announced that more than 59 million Americans with Medicare Part B, including those enrolled in a Medicare Advantage plan, now have access to FDA approved, authorized, or cleared over-the-counter COVID-19 tests at no cost. People with Medicare can get up to 8 tests per calendar month from participating pharmacies and health care providers for the duration of the COVID-19 public health emergency.
People with Medicare Part B (including those enrolled in a Medicare Advantage plan) will now have access to up to 8 FDA-approved, authorized or cleared over-the-counter COVID-19 tests per month at no cost. You may have to show your red, white & blue Medicare card to get the tests.
Compliance Portal® Feature – License Verification
On the home page of the Compliance Portal®, at the very bottom, there is a section called “Compliance Tools & Resources.” License Verification. This feature has been in the portal for at least ten years, and when used properly, you never have to worry about a license or policy expires without knowing about it. To enter a new item, select Create Verification; enter the following: license type: Pharmacist, Orthotic Fitter, Certificate of Liability Insurance; Name on the document; State or Agency; License or Policy Number; Expiration Date, and you can upload the document. Your Compliance Officer at your facility will be notified by email and on the home page when the document is 90 days out and again when it is 30 days to the expiration date. There are two reports available on this page. A License Report is a complete list of all entries and can be sent to whichever agency, or insurance company requires this information. The second report is the Certification of Pharmacy Compliance and credentialing. This document provides the PSAO, PBM, and Wholesaler with very detailed information on the programs you have in place.
CBD Expert Advice for Getting Started
RJ Hedges mail every client of our this new book that will complement and assist the pharmacists, physicians, and chiropractors with understanding CBD and a tool for patients, new and old, to understand what to look for when purchasing CBD drug interactions, and legal requirements. The CBD book is to go to the Owner or Pharmacist-In-Charge; if they don’t have it, check with the staff who manages the mail. This book is designed to help you with CBD sales and make the pharmacy a profit.
R.J. Hedges Bulletin Releases/Webinars
(found in the Message Center)
The following items can be accessed through the Message Center on the Compliance Portal®:
-
April Task List (04/01/2022)